Introduction to Antibody Affinity
Antibodies are critical tools in both research and clinical diagnostics, where their ability to bind specific antigens underpins their utility in various assays. One of the most crucial factors influencing the performance of these assays is antibody affinity. Antibody affinity refers to the strength of the interaction between an antibody's antigen-binding site and its corresponding antigen. This binding strength plays a significant role in determining the sensitivity, specificity, and overall accuracy of diagnostic tests.
The Biophysics of Antibody-Antigen Interactions
Antibody affinity is governed by the principles of molecular recognition. The interaction between an antibody and an antigen is mediated by non-covalent forces, including hydrogen bonds, van der Waals forces, electrostatic interactions, and hydrophobic interactions. The strength of these interactions is quantified as the affinity constant (Ka), which is the ratio of the rate of association (binding) to the rate of dissociation (unbinding).
Ka=konkoff\text{Ka} = \frac{k_{on}}{k_{off}}Ka=koffkon
- k_on: The rate at which the antibody and antigen come together to form a complex.
- k_off: The rate at which the antibody-antigen complex dissociates back into its components.
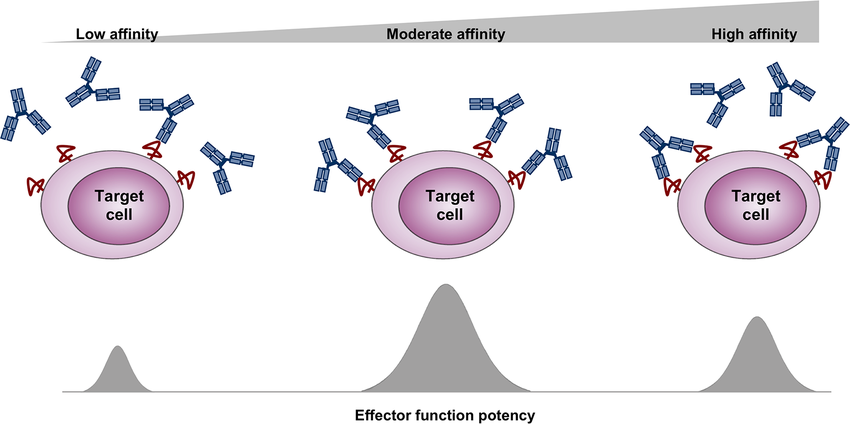
A higher Ka value indicates a stronger affinity, meaning the antibody binds more tightly to the antigen and is less likely to dissociate.
Affinity vs. Avidity: Clarifying the Concepts
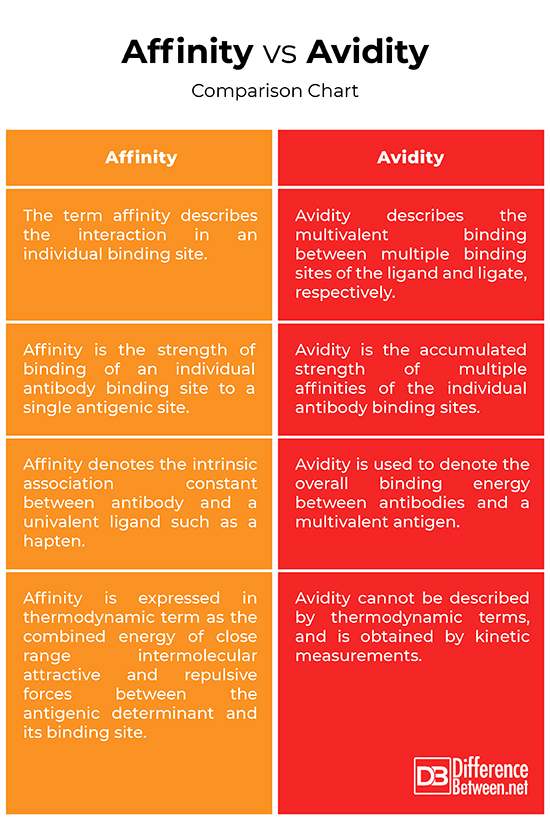
While affinity refers to the binding strength of a single antigen-binding site on an antibody, avidity is a related concept that describes the overall binding strength of an antibody with multiple binding sites (e.g., IgG with two sites, IgM with ten). Avidity takes into account the combined strength of all interactions, which can be significantly higher than the affinity of a single site due to the multivalent binding. High avidity can compensate for lower individual affinities, leading to a more stable antibody-antigen complex.
Impact of Antibody Affinity on Diagnostic Assays
Antibody affinity directly impacts the performance of various immunoassays, including ELISA, Western blotting, and immunohistochemistry. Here’s how:
- Sensitivity:
- High-affinity antibodies are more likely to detect low concentrations of antigens, making the assay more sensitive. In clinical diagnostics, this sensitivity is crucial for early disease detection, where antigen levels might be minimal.
- Low-affinity antibodies might fail to detect these low levels, resulting in false negatives.
- Specificity:
- High-affinity antibodies bind more selectively to their target antigen, reducing the likelihood of cross-reactivity with non-target antigens. This specificity is essential for minimizing false positives in diagnostic tests.
- In contrast, low-affinity antibodies may bind non-specifically, leading to inaccurate results.
- Dynamic Range:
- The dynamic range of a diagnostic assay—the range of antigen concentrations over which the assay can accurately measure—is also influenced by antibody affinity. High-affinity antibodies can extend this range, allowing for more precise quantification across a broader spectrum of antigen levels.
Optimizing Antibody Affinity for Diagnostic Use
The process of optimizing antibody affinity involves several strategies:
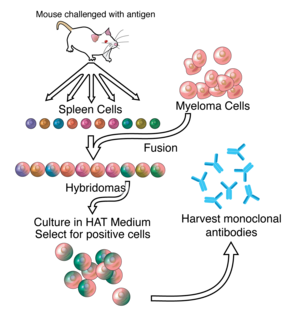
- Hybridoma Technology:
- Antibody Engineering:
- Advances in molecular biology allow for the engineering of antibodies with enhanced affinity. Techniques such as phage display and site-directed mutagenesis enable the modification of the antibody's antigen-binding domain to increase its affinity.
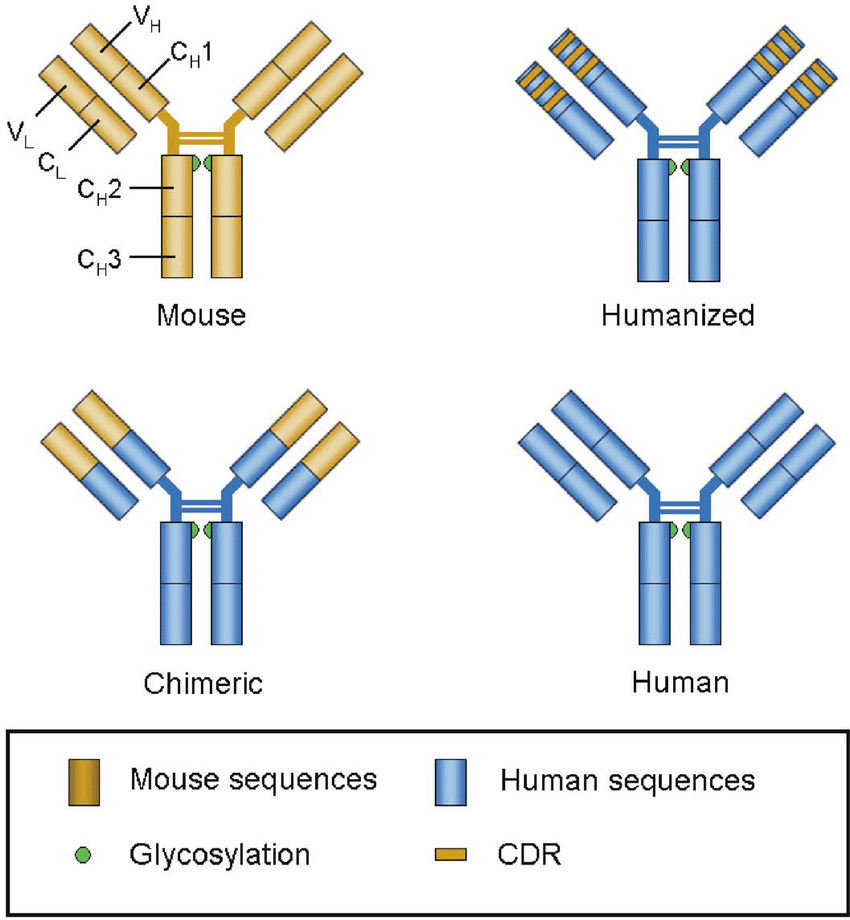
- Advances in molecular biology allow for the engineering of antibodies with enhanced affinity. Techniques such as phage display and site-directed mutagenesis enable the modification of the antibody's antigen-binding domain to increase its affinity.
- Affinity Maturation:
- This natural process can be mimicked in vitro to improve antibody affinity. By subjecting antibodies to repeated rounds of mutation and selection, researchers can achieve progressively higher affinities.
Case Studies: Antibody Affinity in Diagnostic Applications
- HIV Testing:
- In HIV diagnostics, high-affinity antibodies are essential for the early detection of viral antigens. These antibodies can bind to HIV antigens even when present in very low concentrations, allowing for earlier diagnosis and treatment initiation.
- Cancer Biomarker Detection:
- In oncology, the detection of cancer biomarkers such as PSA (Prostate-Specific Antigen) or HER2 is heavily dependent on antibody affinity. High-affinity antibodies improve the sensitivity and accuracy of these tests, leading to better patient outcomes.
- Pregnancy Tests:
- Pregnancy tests are based on the detection of hCG (human chorionic gonadotropin) in urine or blood. High-affinity antibodies ensure that even minute quantities of hCG are detected, providing reliable and early confirmation of pregnancy.
Challenges and Considerations
While high-affinity antibodies offer numerous advantages, there are potential challenges:
- Affinity-Related False Positives:
- Extremely high-affinity antibodies may sometimes bind to structurally similar non-target antigens, leading to false positives. Balancing affinity with specificity is crucial in assay design.
- Production Costs:
- Engineering and producing high-affinity antibodies can be cost-intensive, which may influence the overall cost of diagnostic tests.
- Batch-to-Batch Variability:
- Consistency in antibody affinity across production batches is critical for reliable diagnostics. Variability can lead to inconsistent assay performance.
Antibody affinity is a key determinant of diagnostic accuracy, influencing both the sensitivity and specificity of immunoassays. Understanding and optimizing affinity is essential for developing reliable diagnostic tools that can accurately detect and quantify antigens. As technology advances, the ability to engineer antibodies with precise affinities will continue to enhance the performance of diagnostic tests, ultimately leading to better clinical outcomes.